When using either epoxy or polyester resins in the production of soil thin sections it is essential to remove as much water from the soil to allow the hydrophobic resin to fill all the pore spaces effectively. This can be done either by air or oven drying or by replacing the water with another liquid with which the resin is miscible, acetone (propanone) being the usual choice. The water/acetone exchange can be performed either in the liquid phase, or in the vapour phase however, at Stirling we only use vapour phase drying. This reduces the dissolution of organic material, avoids undue disturbance and does not saturate the sample. We only employ air or oven drying on very rare occasions as this method usually produces too much shrinkage or cracking of the sample.
 The acetone vapour drying process involves removing both of the lids from the tin, replacing the bottom one with a perforated lid to allow the free flow of acetone vapour, then placing the soil sample on a raised platform above a bath of acetone. A container of anhydrous calcium chloride can also be added to facilitate drying. The acetone is changed every three or so days.
The acetone vapour drying process involves removing both of the lids from the tin, replacing the bottom one with a perforated lid to allow the free flow of acetone vapour, then placing the soil sample on a raised platform above a bath of acetone. A container of anhydrous calcium chloride can also be added to facilitate drying. The acetone is changed every three or so days.  Over a period of time most of the water will be removed from the soil sample and be replaced by acetone. It is important to be able to monitor water removal to ascertain when sufficient water has been removed from the sample. Methods for monitoring of water removal employed by other workers have included; NMR spectroscopy, (Murphy 1), solubility in petroleum spirit, (Fitzpatrick 2 ); and enthalpimetry, (Moran & McBratney 3). The densimetric method used at Stirling was developed by Muriel MacLeod and is cheap, accurate and easily carried out. Solvent guide tables do not give detailed values between 0 – 10% water in acetone mixtures, therefore standards were produced from which calibration graphs could be drawn (see table image below). Standards were made, using calibrated pipettes, as follows: 0 cm3 to 10 cm3 (in 1 cm3 increments) of water made up to 100 cm3 in volumetric flasks with acetone. However, these standards are v/v, all comparable data are expressed in w/w. Since the specific gravity of acetone at 20°C is 0.7911 then 1 cm3 of acetone will weigh 0.7911 g at this temperature. The specific gravity of water at 20°C can be taken as 1.0 Therefore all v/v data can be converted to w/w data, and this can be repeated at various temperatures
Over a period of time most of the water will be removed from the soil sample and be replaced by acetone. It is important to be able to monitor water removal to ascertain when sufficient water has been removed from the sample. Methods for monitoring of water removal employed by other workers have included; NMR spectroscopy, (Murphy 1), solubility in petroleum spirit, (Fitzpatrick 2 ); and enthalpimetry, (Moran & McBratney 3). The densimetric method used at Stirling was developed by Muriel MacLeod and is cheap, accurate and easily carried out. Solvent guide tables do not give detailed values between 0 – 10% water in acetone mixtures, therefore standards were produced from which calibration graphs could be drawn (see table image below). Standards were made, using calibrated pipettes, as follows: 0 cm3 to 10 cm3 (in 1 cm3 increments) of water made up to 100 cm3 in volumetric flasks with acetone. However, these standards are v/v, all comparable data are expressed in w/w. Since the specific gravity of acetone at 20°C is 0.7911 then 1 cm3 of acetone will weigh 0.7911 g at this temperature. The specific gravity of water at 20°C can be taken as 1.0 Therefore all v/v data can be converted to w/w data, and this can be repeated at various temperatures 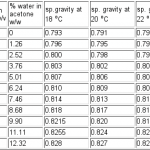 (see table) In all cases the specific gravity of water was assumed to be 1.00 as the variability over the 4 degree range at such low concentrations would have little effect.
(see table) In all cases the specific gravity of water was assumed to be 1.00 as the variability over the 4 degree range at such low concentrations would have little effect.
Keeping the temperature constant at 20°C in a thermostatically controlled water bath, the specific gravity of solutions over the range 1% to 10% water in acetone was measured. This produced a calibration graph for 20°C ambient temperature. The process was repeated at 18°C and 22°C, these being the most probable ambient temperatures of the acetone baths.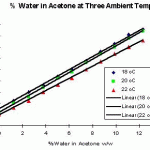
Experience has shown that one need only start to monitor the water content of the acetone in the baths after about 6 changes of acetone. This can be done very simply by removing about 100 cm3 of the acetone into a measuring cylinder, recording the temperature, and the  specific gravity by use of a 0.790 – 0.800 range hydrometer. The water content can then be calculated by reference to the calibration graph for the recorded temperature. At the point when the water content reaches 0.5% or less the soil samples are then ready for impregnation with resin. The method has been in use now for several years and has proven to be quick, easy, very inexpensive and, above all, reliable.
specific gravity by use of a 0.790 – 0.800 range hydrometer. The water content can then be calculated by reference to the calibration graph for the recorded temperature. At the point when the water content reaches 0.5% or less the soil samples are then ready for impregnation with resin. The method has been in use now for several years and has proven to be quick, easy, very inexpensive and, above all, reliable.
References:
(1) Murphy, C.P. (1986) Thin Section Preparation of Soils and Sediments. AB Academic Publishers, Berkhamsted.
(2) Fitzpatrick, E.A. (1984) Micromorphology of Soils. Chapman and Hall Ltd.
(3) Moran, C.J. and McBratney, A.B. CSIRO Divisions of Soils, Technical Memorandum 13/88 “A method for dehydration and impregnation of clay soil”.

2 Pingbacks